Clinical drug development involves a multi-phase process, beginning with preclinical studies, advancing through Phase I, II, and III trials, and culminating in regulatory submissions. Imaging plays a pivotal role at each stage, particularly in oncology trials, where traditional tumor size measurement often does not fully capture the effects of novel therapies, such as targeted treatments and immunotherapies.
- Phase I Clinical Trials: The initial trials focus on safety, pharmacokinetics, and pharmacodynamics. Imaging techniques such as MRI and PET are employed to assess early tumor responses, detect signs of anti-tumor activity, and evaluate changes in tissue perfusion, metabolic activity, or cellularity.
- Phase II Clinical Trials: These trials aim to assess efficacy and safety in a targeted patient population. While tumor size measurements based on RECIST (Response Evaluation Criteria in Solid Tumors) remain a standard method for evaluating response, functional imaging like dynamic contrast-enhanced MRI (DCE-MRI) and diffusion-weighted MRI (DW-MRI) offer more predictive insights into tumor biology, providing early indicators of therapy effectiveness.
- Phase III Clinical Trials: The final phase involves larger patient cohorts and the submission of results for regulatory approval. Here, imaging not only provides evidence of treatment efficacy but also helps assess survival rates, progression-free survival, and other long-term outcomes.
Types of Imaging Techniques
Morphological Imaging (CT and MRI): Standardized methods to measure tumor size, which is the primary criterion for evaluating drug response in early-stage trials.

Morphological Imaging (CT and MRI): Standardized methods to measure tumor size, which is the primary criterion for evaluating drug response in early-stage trials.
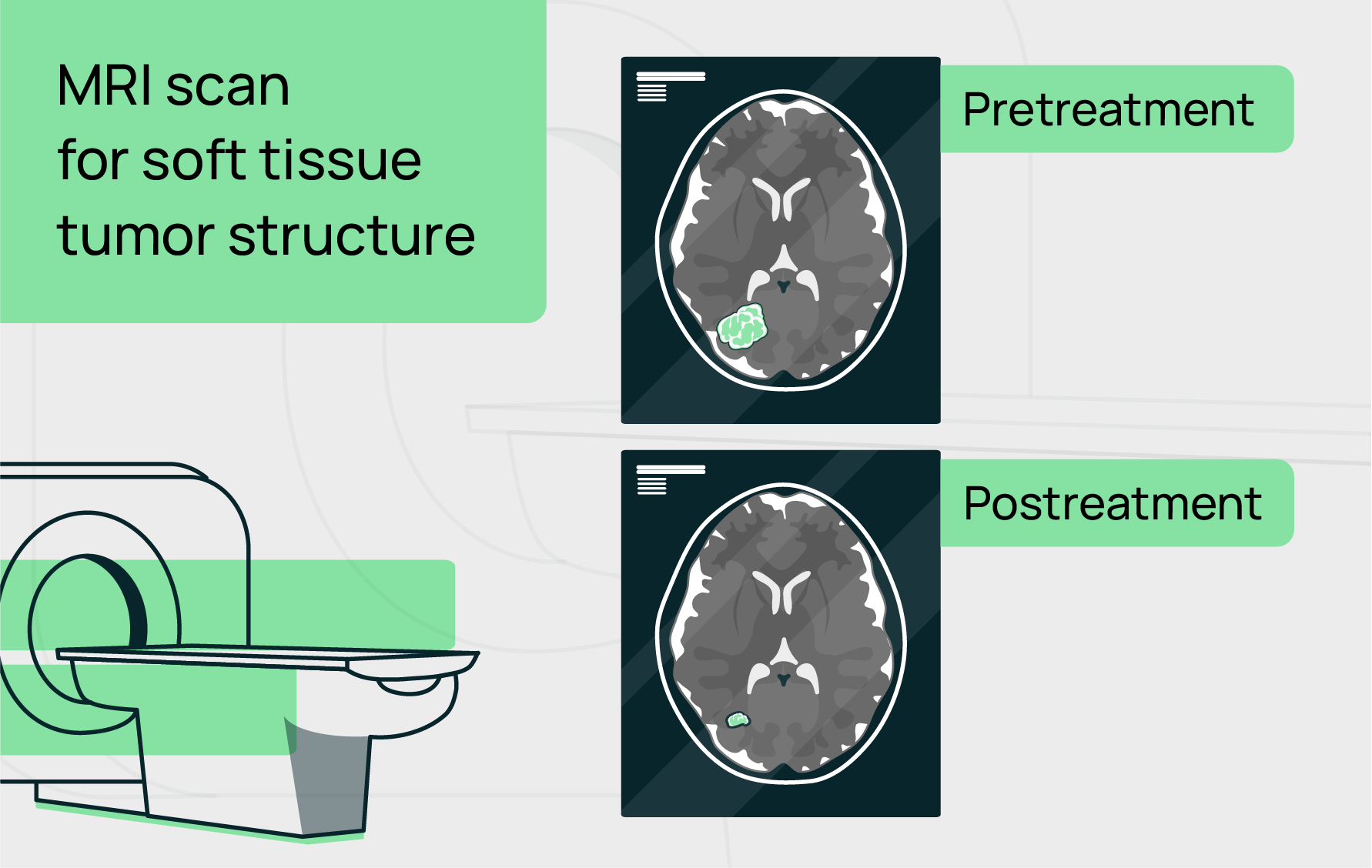
Functional Imaging (PET/CT, DCE-MRI, DW-MRI): These techniques assess aspects of tumor physiology, such as blood flow, metabolism, and cellularity, which can provide earlier and more precise biomarkers for treatment response.
Functional Imaging (PET/CT, DCE-MRI, DW-MRI): These techniques assess aspects of tumor physiology, such as blood flow, metabolism, and cellularity, which can provide earlier and more precise biomarkers for treatment response.
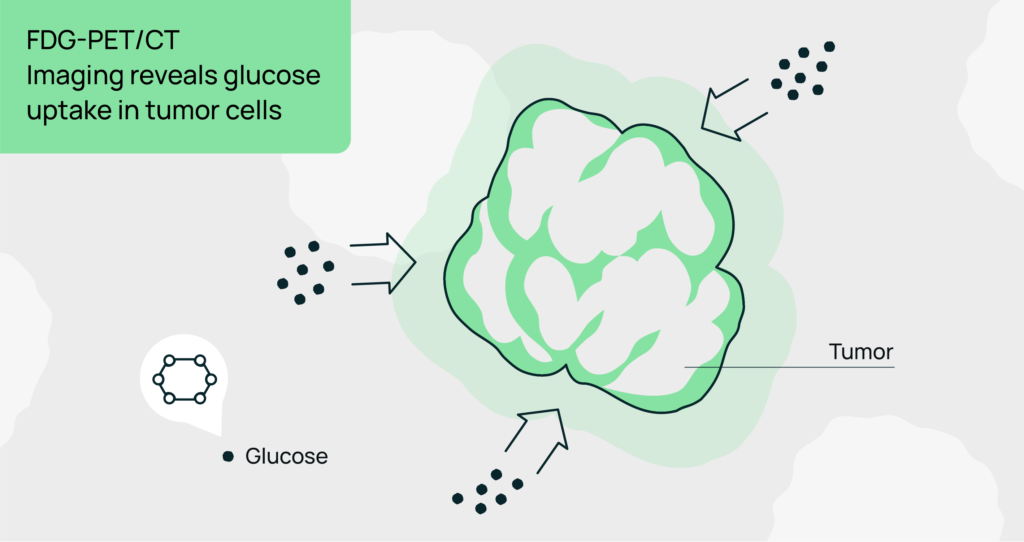
FDG-PET/CT: This imaging modality measures glucose metabolism, which is often elevated in tumor cells. It can detect early metabolic changes even before tumor shrinkage is visible on traditional imaging, thus providing predictive information about treatment response.
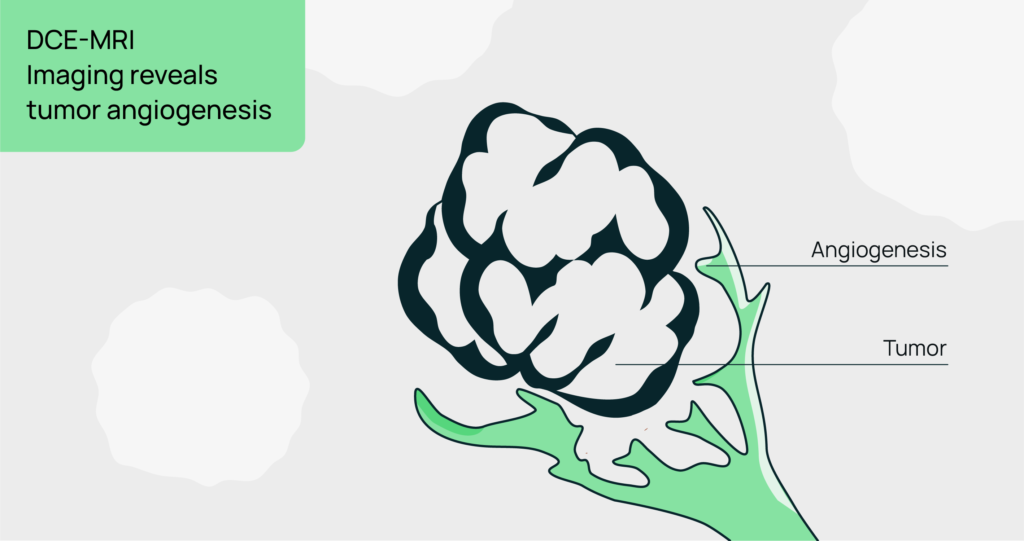
DCE-MRI: A functional imaging technique that evaluates tissue perfusion and vascular permeability, offering detailed insights into tumor angiogenesis and therapy-induced changes in vascular properties.
DW-MRI: This technique assesses cellularity and tissue structure by measuring the movement of water molecules. Changes in diffusion properties can indicate the effectiveness of chemotherapy or novel therapeutic agents before size reduction is evident.
Imaging Biomarkers in Oncology Clinical Trials
Imaging biomarkers (IBs) are defined as measurable indicators derived from in vivo medical images, reflecting biological processes, disease states, or responses to treatment. Their application in oncology trials is particularly valuable for diagnosing, staging, predicting treatment efficacy, and monitoring therapeutic response.
Applications of Imaging Biomarkers:
- Prognostic Biomarkers: Used to predict overall clinical outcomes, such as survival or progression-free intervals.
- Predictive Biomarkers: Aid in assessing the likelihood of response to specific therapies.
- Surrogate Endpoints: Serve as substitutes for direct clinical endpoints (e.g., tumor progression).
Standardization
Variability in imaging techniques, equipment, and analysis across trial sites complicates reproducibility.
Data Interpretation
Sophisticated analysis pipelines and robust quality assurance protocols are required.
Regulatory Hurdles
Demonstrating clinical utility and cost-effectiveness for widespread implementation.
Despite its promise, the use of imaging in clinical trials faces challenges, including the standardization of techniques across multiple centers, the high cost of advanced imaging, and the complexity of data interpretation. For example, DCE-MRI and DW-MRI require careful calibration and centralized analysis to ensure consistency and reliability across diverse trial sites. However, as research progresses and technology improves, these imaging techniques hold the potential to become even more integral to drug development, offering predictive insights that go beyond traditional size measurements.