Artificial intelligence is changing the way we approach healthcare, especially breast cancer. It’s helping doctors detect and treat diseases more effectively than ever before. AI breast cancer innovations have enhanced early detection accuracy, optimized treatment plans, and alleviated workloads for medical professionals.
This article explores how AI reshapes breast cancer detection and treatment, from diagnostic tools to advanced therapeutic solutions.
AI breast cancer detection
Traditional breast cancer detection relies on mammograms, MRIs, and ultrasounds, all of which require the expertise of skilled radiologists. However, these methods have limitations, such as false positives and negatives. AI is stepping in to address these issues. AI breast cancer detection uses advanced machine learning algorithms to analyze vast datasets, improving diagnostic accuracy.
- AI in mammogram analysis
AI has proven particularly effective in mammogram interpretation. Deep learning algorithms can detect subtle patterns that the human eye may overlook. In fact, it has been proven that AI systems can reduce error rates in breast cancer detection. Additionally, AI-powered computer-aided detection (CAD) programs improve tumor identification accuracy and help differentiate between benign and malignant lesions, minimizing the number of unnecessary biopsies.
- AI in MRI interpretation
Another significant advancement is the use of AI in MRI analysis. MRIs provide more detailed views of soft tissues than mammograms, but the total data they generate can be overwhelming. AI breast cancer detection models applied to MRI scans process large amounts of information swiftly and accurately, identifying abnormalities faster and more effectively than traditional methods.
Another significant advancement is the use of AI in MRI analysis. MRIs provide more detailed views of soft tissues than mammograms, but the total data they generate can be overwhelming. AI breast cancer detection models applied to MRI scans process large amounts of information swiftly and accurately, identifying abnormalities faster and more effectively than traditional methods.
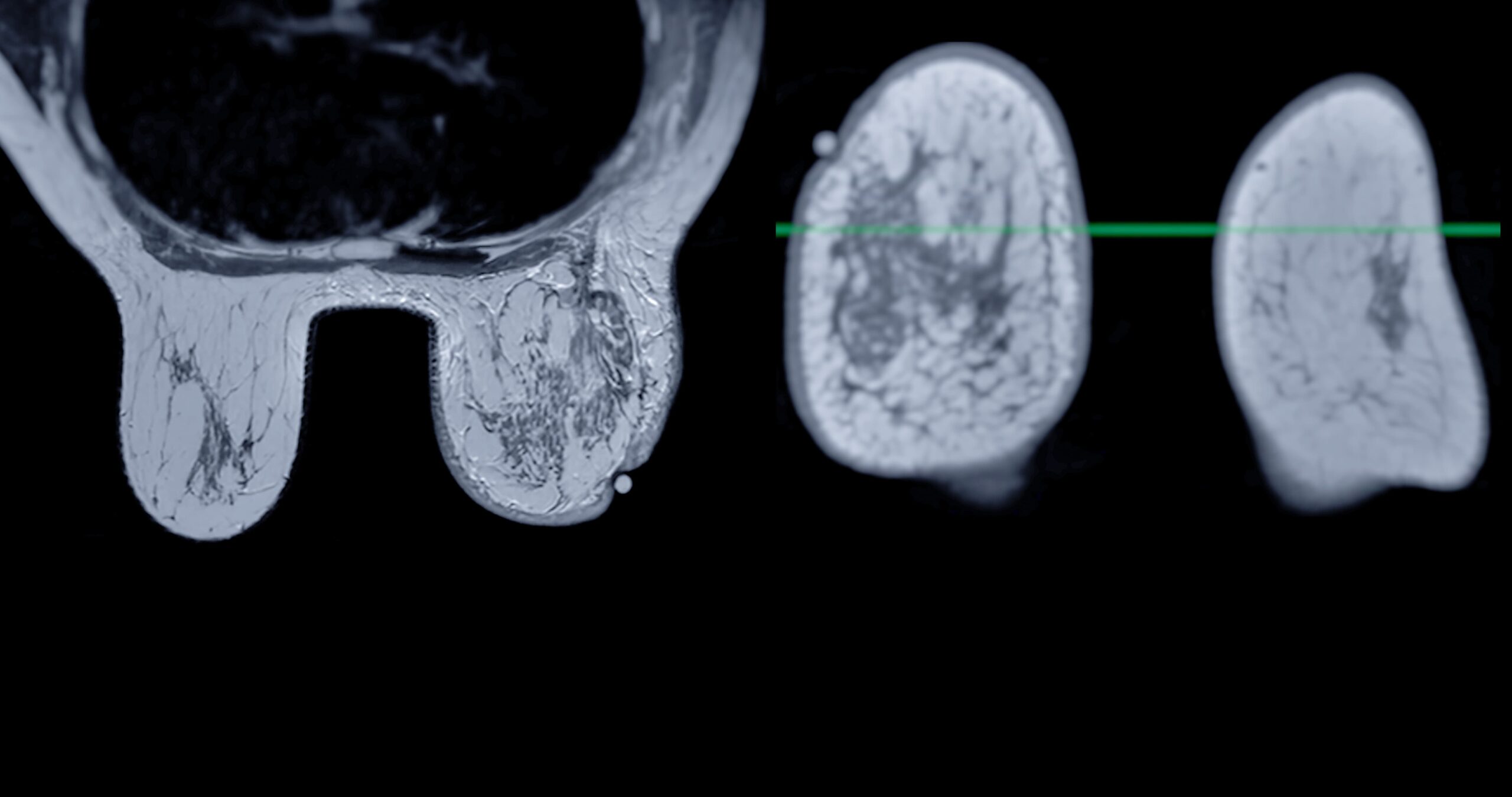
AI treatment for breast cancer
Once breast cancer is diagnosed, treatment must be carefully personalized to maximize the chances of success. AI is also a valuable tool at this stage, assisting doctors in personalizing therapies based on the tumor’s characteristics and the patient’s genetic profile.
Personalized therapies with AI
AI-driven treatments for breast cancer are reshaping the future of precision medicine, enabling more personalized and targeted therapeutic approaches.
AI analyzes the tumor’s genetic and molecular data and the patient’s medical history to recommend personalized treatments. This approach is particularly beneficial for triple-negative breast cancers, which often do not respond well to standard therapies. AI enables oncologists to identify more effective drug combinations and develop targeted therapies that consider specific genetic mutations within the tumor.
Optimizing treatment plans with AI
AI also assists in predicting how a tumor will respond to different therapies. Predictive models can analyze historical data on the effectiveness of chemotherapy, radiotherapy, and immunotherapy, suggesting modifications based on the tumor’s progression. This real-time adjustment capability can significantly improve survival rates while reducing side effects.
AI in breast cancer surgery
AI is also making strides in surgical planning. AI-powered algorithms are being trained to accurately identify tumor margins during breast-conserving surgeries, reducing the likelihood of residual cancerous tissue. This minimizes the risk of recurrence and improves long-term outcomes.
Challenges and the future of AI in breast cancer
While AI has demonstrated tremendous potential in breast cancer detection and treatment, there are still challenges. The accuracy of AI models depends heavily on the quality and quantity of data available. Moreover, integrating AI technologies into everyday clinical practice requires significant investments in tech infrastructure and training for healthcare professionals.
Looking ahead, the adoption of AI breast cancer technologies in clinical settings is expected to grow to enhance diagnostic precision and treatment personalization, optimize hospital workflows, and reduce costs. Ongoing research explores AI’s potential to identify hereditary risk factors and monitor patients’ long-term responses to treatment.
Artificial intelligence is transforming every aspect of breast cancer diagnosis and treatment. Advances in AI breast cancer detection are reducing diagnostic errors and enabling earlier detection, while innovations in AI treatment for breast cancer facilitate more personalized and optimized therapies. Though progress still needs to be made, the future of breast cancer care with AI looks promising, and these technologies are likely to become a fundamental part of oncology in the future.